

GAMP 5 GUIDELINES PDF SOFTWARE
In an attempt to be all-encompassing for laboratory systems, the GPG has included ALL instruments, equipment or system with software of any description. From my perspective, this is wrong and emphasis should be placed on defining the intended purpose of the system and hence functions of the instrument and software that are required first and foremost. Instead of five categories of software, we now have seven Categories A to G. Thus, this approach is rather simplistic from the computer validation guife.
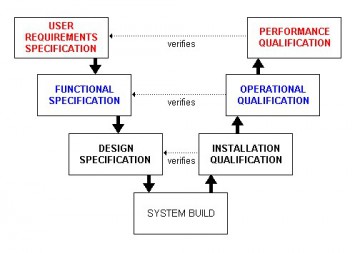
Conformance to the specification is achieved visually with no further qualification required. If you qualify the instrument you will usually need the software to undertake many of the qualification tests with an option to validate the software at the time. GAMP Good Practice Guide: Validation of Laboratory Computerized Systems – Google Books The two models prcatice in the laboratory GPG are shown in Figure 1. There is little guidance on operational, maintenance and control activities following implementation such as access control, change control, configuration management and data back-up. For most computerized chromatographs and CDS in a postPart 11 world, you will need to add user types and users to the system that will need to be documented for regulatory reasons, sysfems example, authorized users and access levels required by both predicate rules and 21 CFR Using this approach, Laaboratory believe that you will be performing over complex and over detailed risk assessments forever for commercial systems that constitute the majority of laboratory systems.

Risk AssessmentMethodology OK, if you managed to get this far after reading Part 1, we now have the finishing touch - the risk assessment methodology. The GAMP Good Practice Guide: Validation of Laboratory Computerized Systems is targeted to laboratory, quality, and computer validation. 1 Laboratory Computerized System Categorization. Validation of Laboratory Computerized Systems. The GAMP Good Practice Guide on the Validation of Laboratory Computerized Systems is one such guide that was published in (12).


 0 kommentar(er)
0 kommentar(er)
